一、超高真空常用单位
1. 毫巴(mbar)来自于气压的单位bar,1000 mbar = 1 bar = 1*105 Pa;
2. 托(Torr)来自于托里拆利实验中的毫米汞柱(mmHg),760 Torr =1 atm;
3. 帕(Pa)来自于国际单位制(SI),1 Pa = 1 N/m2;
备注:Pa是国际单位制中的导出单位而非基本单位
备注:1 bar严格定义为105 Pa, 1 atm严格定义为101325 Pa,两者在实际使用中基本认为一致,但定义上有所不同。
备注:在实际使用中,由于Torr和mbar数值相近,对不要求精度时一般认为等价。
备注:工程上经常会使用公斤(kg/cm2)作为压力的单位,数值上接近于105 Pa。
二、超高真空的定义
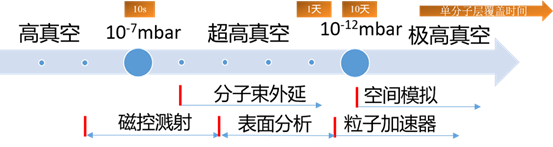
1. 超高真空(ultra-high vacuum,UHV)一般定义为10-7-10-12 mbar;
2. 高真空(high vacuum,HV)一般定义为 > 10-7 mbar;
3. 极高真空(Extreme high vacuum,XHV)一般定义为 < 10-12 mbar。
三、超高真空的特点
1. 洁净度高
高洁净度是表面分析需要用到超高真空的根本原因。表面物理研究的往往是表面几个原子层的物理现象,因此,即便是在真空条件下,气体分子在样品表面的吸附往往会显著影响实验结果。我们经常用“寿命(lifetime)”来描述样品表面从清洁到所受污染影响实验结果所花费的时间。不同样品之间,由于对气体分子吸附能力的不同,样品寿命有很大差异。即使对于同种样品,不同实验对于样品寿命也会有完全不同的定义。通常来说,表面态的寿命比体态的寿命要短得多。
表面科学中用L(Langmuir)定义样品表面的暴露情况,1 L = 10-6 Torr*s。我们可以看到,样品的暴露情况和气压是成反比关系。所以,为了提高样品的寿命,我们往往会尽可能地提高系统的真空度。
如果以室温条件下的N2分子进行计算,考虑所有碰撞表面的分子全部被吸附的话,在10-6 Torr的真空条件下,3秒钟会在样品表面吸附一层分子。在科普宣传中我们经常以10-6 Torr对应1 s单分子层覆盖时间来描述真空的重要性,这个提法比较形象,易于理解,但从事表面研究的同学们一定不要以此作为科学研究的依据。
2. 平均自由程长
每个气体分子相邻两次碰撞的距离统计平均值称为分子的平均自由程。分子平均自由程的大小和真空中分子的种类、密度和运动速度都有关系。在常温下,考虑N2的话,气体分子的平均自由程和气压成反比:大气压(105 Pa)下,平均自由程为59 nm,10-7 Pa的平均自由程则高达59 km。根据这一参数,我们可以估计磁控溅射生长所需要的最低真空度。
电子平均自由程指的是电子和气体分子连续两次碰撞之间所走过的路程统计平均值(忽略电子之间的碰撞)。这一参数主要应用于光电能谱实验系统。
3. 绝热性
超高真空条件下,一般忽略热对流,主要考虑热辐射和热传导。低温系统(液氦、液氮)主要考虑阻止外界热量的传入。对使用液氮的系统来说,热传导是主要的热量来源;对使用液氦的系统来说,外部热辐射是不可忽略的,在设计系统时要特别注意。高温系统则需要考虑加热灯丝产生热辐射带来的材料升温放气。高温下热传导主要对热偶的温度测量产生影响。此外,材料被加热到较高温度后,自身产生的热辐射也不可忽略。
四、超高真空的应用领域
超高真空应用领域非常广泛,这里我们列举了和表面物理研究关系最密切的几种,包括磁控溅射、激光脉冲沉积、分子束外延、表面分析和粒子加速器。
分子束外延和表面分析领域广泛使用超高真空技术,各种类型的分子束外延设备、光电子能谱和扫描隧道显微镜等制备表征系统都在这个范围内工作。由于真空系统在系统建设成本中往往占据相当大的比例,如何选择合适的泵组并通过恰当的方式迅速获得尽可能好的真空度,是困扰相关领域的普遍问题。
粒子加速器对真空要求最为苛刻,但因为整体系统造价较高,真空泵组不是成本的主要构成,一般尽可能配置较好的真空泵,加之加速器的腔体内一般没有污染源,真空度通常会达到极高真空的范围。
磁控溅射因为机制问题,蒸镀过程中产生的污染较大,通常不会追求特别高的真空度,一般用分子泵组即可满足使用条件。近年来,随着技术的不断进步和研究需求的进一步发展,磁控溅射系统的真空度持续提高,超高真空相关技术也在不断进入这一领域。
激光脉冲沉积(PLD)技术过去对真空度的需求介于分子束外延和磁控溅射之间,近年来由于与分子束外延(MBE)技术逐渐融合,真空度要求也在不断提高。激光分子束外延(LMBE)就是在PLD中融入了MBE的超高真空技术。
I. Common units for pressure
1. mbar comes rom the unit bar, 1000 mbar = 1 bar =1 x 105 Pa;
2. Torr comes from the mmHg in the Torricelli’s experiment, 760 Torr = 1 atm;
3. Pa from the International System of Units (SI), 1 Pa = 1 N/m2.
Note: Pa is an SI derived unit.
Note: 1 bar is strictly defined as 105 Pa and 1atm is strictly defined as 101325 Pa, which is generally considered to be the same in practice, but different in definition.
Note: In practice, the value of torr and mbar are generally considered equivalent when accuracy is not required.
II. The definition of UHV
1. UHV is generally defined as 10-7-10-12mbar;
2. High vacuum (HV) is generally defined as > 10-7 mbar;
3. Extreme high vacuum (XHV) is generally defined as < 10-12 mbar.

III. The characteristics of UHV
1. High cleanliness
Surface physics focused on the physical phenomena of several atomic layers on the surface. Even in high vacuum, the adsorption of gas molecules on the surface of the sample tends to significantly affect the experimental results. We often use "lifetime" to describe the time it takes for the clean sample surface to get contaminated. The "lifetime" varies greatly from sample to sample, due to the different adsorption capacity of the gas molecule. Even for the same sample, different experiments may have completely different definitions of "lifetime". In general, the "lifetime" of the surface state is much shorter than that of the body.
Surface science uses Langmuir (L) to define the exposure of sample surface, 1 L = 10-6 Torr*s. We can see that the exposure of sample surface is proportional to the air pressure. Therefore, in order to increase "lifetime" of the sample, we tend to improve the vacuum of the system as much as possible.
If calculated at room temperature and N2 molecules, considering that all molecules on the collision surface are all adsorbed, a layer of N2 molecules would be formed on the sample surface within 3 seconds in 10-6 Torr. In popular science propaganda, we often describe the importance of vacuum by “10-6 Torr corresponding to a single-molecule layer coverage time of 1 s”, which is more visual and easier to understand. However, for students who engaged to surface science, you should note that it is not an accurate description.
2. Mean free path
The statistical average distance traveled between two collisions is called mean free path. Mean free path can be affected by the type, density and speed of molecules in the vacuum. At room temperature, consider N2, the mean free path is proportional to the pressure: the mean free path is 59 nm in 105 Pa and reaches to 59 km in 10-7 Pa. Based on this parameter, we can estimate the minimum vacuum required for Physical vapor deposition (PVD).
The mean free path of electrons refers to the statistical average of the distance traveled between two consecutive collisions with electrons or gas molecules. This parameter is mainly applied to the photoelectron energy spectrum systems.
3. Insulation
Under UHV condition, thermal convection is generally ignored, mainly considering thermal radiation and thermal conduction. Low temperature systems (such as liquid helium, liquid nitrogen) are mainly considered to prevent the introduction of external heat. For systems that use liquid nitrogen, thermal conduction is the main source of heat, and for systems that use liquid helium, external thermal radiation is not negligible and should be designed with care. For high temperature systems, the outgassing of materials caused by the heating from filament should be considered. At high temperature, heat conduction can significantly affect the temperature measurement of thermocouple. In addition, the heat radiation generated by the material after it is heated to a high temperature cannot be ignored.
IV. Applications of UHV systems
UHV is widely used in many fields. Here we list some technologies that are most closely related to the study of surface physics, including magnetron sputtering, pulsed laser deposition (PLD), molecular beam epitaxy (MBE), surface analysis and particle accelerator.
UHV technology is widely used in the field of MBE and surface analysis. Many preparation and characterization systems such as MBE, photoelectron spectrometer and scanning tunnel microscopes work in UHV condition. Since vacuum systems often account for a considerable proportion of the cost of system construction, how to choose the right pump set and quickly obtain the best vacuum is a common problem in the relevant fields.
Particle accelerator vacuum requirements are the most demanding. Since the overall system cost is very high, the vacuum system is not the main component of the cost. Therefore, they usually use the best vacuum pumps. In addition, the accelerator chamber is generally clean without source of pollution. So, particle accelerators usually reach to very high vacuum level, sometimes even in the range of XHV.
For magnetron sputtering, vacuum is limited due to the mechanism: a large pollution was produced in the evaporating process. Therefore, generally with molecular pump group can meet the conditions of use. In recent years, with the continuous progress of technology and the further development of research needs, the vacuum of magnetron sputtering system has been continuously improved, and ultra-high vacuum-related technology has also entered this field.
The demand for vacuum in the past of laser pulse deposition technology is between molecular beam extension and magnetron sputtering. In recent years, the vacuum requirement is constantly increasing due to the gradual fusion with molecular beam extension technology. Laser molecular beam epitaxy (LMBE) is a kind of PLD which integrates the UHV technology of MBE.

